[Interview] Gyeonghun Seo, CEO of E&S Healthcare
With the first blood-based diagnostic kit developed to detect breast cancer, E&S Healthcare aims at becoming a global in-vitro diagnostic company. Our mission is to attain a quick and accurate in-vitro diagnosis for diseases whose early detection is crucial.
Back in 2007, the South Korean government initiated a long-term R&D project for in-vitro diagnosis. In six years, it spent over 10 billion KRW to pursue the development of new diagnostic metabolite biomarkers. The outcome was positive. The government secured patents and technologies. Gyeonghun Seo, CEO and Professor of Pai Chai University, who had led the project, felt it was a pity to lose all the hard work and research done with taxpayers’ money and have only patents and dissertations remain as a legacy.
The same year, he started his own business and then got the in-vitro diagnosis technology transferred from the university. Out of various types of cancer including lung cancer, he especially secured research results optimized for breast cancer. He then developed a diagnostic kit to detect breast cancer using Trx 1, a crucial antioxidant protein inside the cells, as a bio-marker.
The diagnostic kit developed to screen for breast cancer is cheap, quick and convenient. Following its successful export to Malaysia last year, it was also a hit in the markets in Europe, the United States and South America this year. The kit’s excellent performance was recognized overseas.
Mr. Seo is now fully immersed in this business and has resigned as a professor. The developed kit is expected to go through the Phase 3 clinical trial for KFDA approval.

"When compared to existing products, a more accurate and reliable diagnosis"
“Around the globe, one out of eight people suffer from breast cancer. A universality of US local statistics showed that 85 percent of breast cancer patients did not have any family history. This means any woman has a risk of developing the disease. That said, with early detection, and the benefits of treatment the survival rate can go up.”
Mr. Seo emphasizes the needs for early diagnosis of breast cancer. Breast cancer is a major cancer causing death among women. In South Korea, its prevalence rate comes in second, following thyroid cancer. If early detection of breast cancer is possible, the ten-year survival rate can be raised up to about 94 percent.

Mammography is the only screening method certified by the WHO. Despite this method’s high sensitivity, it has shortcomings such as low specificity.
Especially for Asian women including Korean women with dense breasts, mammography has limitations with low sensitivity, which means that women have to go through the process two or three times in order to have a final, definite diagnosis. As a yet another shortcoming, it is also relatively expensive, making it difficult for the ordinary public to use it to its full advantage.
Mr. Seo explained, “There are limitations in the use of mammograms due to Koreans’ physical characteristics, and molecular diagnosis also has limits due to its sole focus on prognosis forecasting and high cost that reaches to more than 4 million KRW.”
He added, “Many companies around the world have set out to develop early diagnosis technology for breast cancer by attracting large-scale investment, but most of them are still in the early R&D stage. They also tend to focus on prognosis and forecasting, like the existing methodology, rather than early detection or screening diagnoses.”
The diagnostic kit “DxMe BC” developed by B&S Healthcare has overcome these limitations. It has a high sensitivity and specificity of 96 percent or higher in terms of the statistical analysis indicators used to evaluate the performance of in-vitro diagnostic kits. It also has the advantage of easy and simple testing, using blood.
A study conducted on normal women and breast cancer patients showed that both positive and negative predictive values had high reliability of 96 percent or higher.
The kit has also demonstrated its usefulness as a tool to complement mammography. The study also showed that cases determined as “normal”, “subject to second testing” or “benign tumor” had been accurately diagnosed as negative and normal. It also boasted of higher accuracy than other breast-cancer diagnostic kits and could be used for diagnosis regardless of age, disease stages or proliferation rate.
The diagnostic kit has maintained consistent testing quality with an automated process.The system has been automated with self-developed fully-automatic diagnosis/analysis devices. Mr. Seo emphasized, “By just putting blood in the kit, the results are generated. Without any errors caused by age or sex, a quick and accurate diagnosis is possible.”
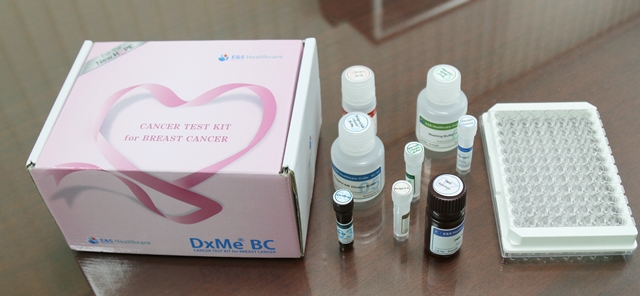
“After starting up my own business, I have worked day and night to create the necessary documentation. I have already completed the procedures to obtain the licenses and permits available in South Korea including certifications for QC and QA. This year, I plan to strengthen the company internally and externally expand the company as well.”
As the kit has hit the markets at home and abroad this year, the company is also externally expanding through additional investments. E&S Healthcare succeeded in attracting investments of 9 billion KRW last August. Participating investors include NHN, Mirae Asset Daewoo, Magellan, BHA Investment Association, E&SL, InterValue and Daedeok Venture Partners.
E&S Healthcare plans to use the investment funds for clinical trials in the United States and South Korea and the establishment of a business infrastructure in the US. It also expects to expand both human resources and networks.
Mr. Seo has regarded this year as a base year for commercialization. One of the most noteworthy nations is Malaysia. Following the first exports to the Malaysian market last year, the company is also pursuing an empirical clinical study under the governmental support. The empirical study is forecast to be carried out in earnest on 520 subjects in cooperation with the University of Malaya Medical Centre and to be completed within the first half of next year.
The company is set to advance into the European market as well. Last year, it had its products registered in the Netherlands, and discussions are underway with a German company regarding sales rights. In addition, a project is being implemented to include the company’s products in the Brazilian Government’s procurement items.
Mr. Seo said, “I am preparing to meet the requirements necessary to make inroads into the US market. Our products were approved by the US FDA as Grade 2 last year, and currently are at the stage of seeking out a clinical trial and marketing partners.”
On the domestic front, the company is in discussions with the KFDA regarding the approval for a clinical trial and seeking a method to utilize the kit as a tool to complement mammography. With this, it has continued a clinical trial at Chungnam National University Hospital.
The company’s ultimate goal is to leap forward as a global diagnostic firm based on this breast-cancer diagnostic kit, using blood. To this end, it intends to expand the use of in-vitro diagnostic kits to cover other female cancers such as ovarian cancer and endometriosis.
Last but not least, Mr. Seo underscored that the company’s technologies, human resources and networks are all “made in Daedeok (Daejeon).” He expressed the company’s goal of creating a new success story in strong collaboration with other regions and bio-medical industries down the road.
“The company would not have secured this technology or developed this much without large-scale national technological development. I would like to make a new success story based on Daedeok’s human and capital resources from start-up all the way to commercialization. I will make efforts to further develop into a global diagnostic firm.”
댓글 정렬